You Are Here
- Home
- Treatments
Treatments for glioblastoma multiforme
Symptomatic therapy

Supportive treatment focuses on relieving symptoms and improving the patient’s neurologic function. The primary supportive agents are anticonvulsants and corticosteroids.
• Historically, around 90% of patients with glioblastoma underwent anticonvulsant treatment, although it has been estimated that only approximately 40% of patients required this treatment. Recently, it has been recommended that neurosurgeons not administer anticonvulsants prophylactically, and should wait until a seizure occurs before prescribing this medication. Those receiving phenytoin concurrent with radiation may have serious skin reactions such as erythema multiforme and Stevens–Johnson syndrome.
• Corticosteroids, usually dexamethasone given 4 to 8 mg every 4 to 6 h, can reduce peritumoral edema (through rearrangement of the blood-brain barrier), diminishing mass effect and lowering intracranial pressure, with a decrease in headache or drowsiness.
Palliative therapy

Palliative treatment usually is conducted to improve quality of life and to achieve a longer survival time. It includes surgery, radiation therapy, and chemotherapy. A maximally feasible resection with maximal tumor-free margins is usually performed along with external beam radiation and chemotherapy. Gross total resection of tumor is associated with a better prognosis.
Surgery
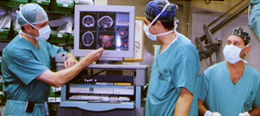
Surgery is the first stage of treatment of glioblastoma. An average GBM tumor contains 1011 cells, which is on average reduced to 109 cells after surgery (a reduction of 99%). It is used to take a section for a pathological diagnosis, to remove some of the symptoms of a large mass pressing against the brain, to remove disease before secondary resistance to radiotherapy and chemotherapy, and to prolong survival.
The greater the extent of tumor removal, the better. Removal of 98% or more of the tumor has been associated with a significantly longer healthier time than if less than 98% of the tumor is removed. The chances of near-complete initial removal of the tumor can be greatly increased if the surgery is guided by a fluorescent dye known as 5-aminolevulinic acid. GBM cells are widely infiltrative through the brain at diagnosis, and so despite a "total resection" of all obvious tumor, most people with GBM later develop recurrent tumors either near the original site or at more distant "satellite lesions" within the brain. Other modalities, including radiation, are used after surgery in an effort to suppress and slow recurrent disease.
Radiotherapy
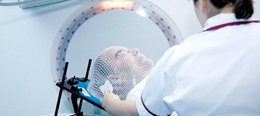
After surgery, radiotherapy is the mainstay of treatment for people with glioblastoma. A pivotal clinical trial carried out in the early 1970s showed that among 303 GBM patients randomized to radiation or nonradiation therapy, those who received radiation had a median survival more than double those who did not.[30] Subsequent clinical research has attempted to build on the backbone of surgery followed by radiation. On average, radiotherapy after surgery can reduce the tumor size to 107 cells. Whole brain radiotherapy does not improve when compared to the more precise and targeted three-dimensional conformal radiotherapy.[31] A total radiation dose of 60–65 Gy has been found to be optimal for treatment.
Chemotherapy
In other cancers where radiation can prolong survival or even cure tumors, the addition of chemotherapy to radiation improves survival over radiation treatment alone. Examples include cervical cancer, throat cancer and others. Because of this, several large clinical trials took place in which it was hoped survival of GBM patients might be improved with the addition of chemotherapy to radiation. Most of these studies showed no benefit from the addition of chemotherapy. However, a large clinical trial of 575 participants randomized to standard radiation versus radiation plus temozolomide chemotherapy showed that the group receiving temozolomide survived a median of 14.6 months as opposed to 12.1 months for the group receiving radiation alone. This treatment regime is now standard for most cases of glioblastoma where the patient is not enrolled in a clinical trial. Temozolomide seems to work by sensitizing the tumor cells to radiation.
The U.S. Food and Drug Administration approved Avastin (bevacizumab) to treat patients with glioblastoma at progression after standard therapy based on the results of 2 studies that showed Avastin reduced tumor size in some glioblastoma patients. In the first study, 28% of glioblastoma patients had tumor shrinkage, 38% survived for at least one year, and 43% survived for at least 6 months without their disease progressing.[38] Unlike the case for colon cancer, lung cancer and other cancers where bevacizumab acts by potentiating chemotherapy, the studies leading to approval showed that in GBM, the addition of chemotherapy to bevacizumab did not improve on results from bevacizumab alone. Bevacizumab reduces brain edema and consequent symptoms, and it may be that the benefit from this drug is due to its action against edema rather than any action against the tumor itself. Some patients with brain edema do not actually have any active tumor remaining, but rather develop the edema as a late effect of prior radiation treatment. This type of edema is difficult to distinguish from that due to tumor, and both may coexist. Both respond to bevacizumab.
Gene transfer
Gene transfer is a promising approach for fighting cancers including brain cancer. Unlike current conventional cancer treatments such as chemotherapy and radiation therapy, gene transfer has the potential to selectively kill cancer cells while leaving healthy cells unharmed. Over the past two decades significant advances have been made in gene transfer technology and the field has matured to the point of clinical and commercial feasibility. Advances include vector (gene delivery vehicle) construction, vector producer cell efficiency and scale-up processes, preclinical models for target diseases and regulatory guidance regarding clinical trial design including endpoint definitions and measurements. In one such approach, researchers at UCLA in 2005 reported a long-term survival benefit in an experimental brain tumor animal model. Subsequently, in preparation for human clinical trials, this technology was further developed by Tocagen Inc., and is currently under clinical investigation in a Phase I/II trial for the potential treatment of recurrent high grade glioma including glioblastoma multiforme (GBM) and anaplastic astrocytoma.
Protein therapeutics

Not so long ago, protein therapeutics were a rarely used subset of medical treatments. Protein therapeutics have increased dramatically in number and frequency of use since the introduction of the first recombinant protein therapeutic — human insulin — in the early 1980s and already have a significant role in almost every field of medicine, including brain cancer. A randomized Phase II clinical study with the protein therapeutic APG101 in Glioblastoma was started at the beginning of 2010. The study compares the efficacy of APG101 in a combined treatment with intravenously administered APG101 together with radiotherapy versus radiotherapy alone. APG101 is a CD95-Fc fusion protein for the treatment of malignant diseases. APG101 constitutes an innovative approach to treating GBM since it has the therapeutic aim to inhibit the invasive growth of glioblastoma cells. This therapy is based on results from the German Cancer Research Center (DKFZ) that in glioblastoma cells, the binding of the CD95-Ligand to its cognate receptor stimulates the invasive growth of the tumor cells. Thus, the inhibition of this interaction by APG101 reduces tumor cell migration. So far, APG101 has been tested in 20 healthy volunteers and 32 patients. It was well tolerated and showed no serious side effects.
Immunotherapy
Relapse of glioblastoma is attributed to the recurrence and persistance of tumor stem cells. In a small trial, a tumor B-cell hybridoma vaccine against tumor stem cells elicited a specific tumor immune reaction thus enhancing immune response to the disease. Larger trials, including tests of different EGFR signaling patterns and their relationship to tumor stem cells being conducted by John A. Boockvar's lab at Weill Cornell Medical College, are in progress to further assess this approach to treating glioblastoma.
Alternating electrical fields
The use of alternating electrical fields to interfere with the division of malignant cells is a new approach to cancer treatment. As opposed to the treatment modalities of surgery, radiation and chemotherapy, which are all used to treat other types of cancer, alternating electrical fields is being explored for the first time in the treatment of glioblastoma. The underlying theory is that in an electrical field of given wavelength, cells attempting to divide will be destroyed. This approach is optimal for brain tumors in that normal brain cells do not divide, but cancer cells within the brain do. GBM patients treated with alternating electrical fields wear electrodes on the scalp attached to the portable Novo-TTF device. The recently released preliminary abstract of a large clinical trial of patients with relapsed GBM showed that treatment with the Novo-TTF device was no better than best supportive care in prolonging survival. A currently open clinical trial for people with newly diagnosed GBM is exploring whether the addition of the Novo-TTF device to standard radiation and temozolomide treatment improves survival over standard treatment alone.
Metabolic therapy

The ketogenic diet has been used successfully to treat glioblastomas in a single case study. Cancer cells have impaired metabolisms and are unable to utilize fats as an energy source. They are nearly completely reliant on glucose, and glutamine to some degree, for their energy. Healthy neurons are able to utilize ketones as extremely efficient energy sources. Removing or greatly restricting carbohydrates from the diet effectively starves the cancer cells without damaging the healthy neurons. In pediatric studies, ketogenic or carb-restricted diets result in a marked decrease in tumor size and growth when used in conjunction with standard therapies. This has not yet been scientifically proven and results may vary for each patient.
Causes of Glioblastoma
For unknown reasons, GBM occurs more commonly in males. Most glioblastoma tumors appear to be sporadic, without any genetic predisposition. No links have been found between glioblastoma and smoking, consumption of cured meat, or electromagnetic fields. Alcohol consumption may be a possible risk factor.
Research About Glioblastoma
- New Gene Changes and Signaling Pathways that Contribute to Glioblastoma Multiforme Development
- The Rembrandt Glioma Dataset
- Four Distinct Subtypes of Glioblastoma
- Genomics and Biology of Glioblastoma Multiforme
- Clinical Implications of TCGA Findings On Glioblastoma
- TCGA Findings on Subtypes of Glioblastoma
- Identifying Important Gene Alterations in Glioblastoma Multiforme
- New Database that Provides Comprehensive View of Glioblastoma Multiforme Genome
- Glioblastoma Multiforme Data Used in the Discovery of a Gene Pathway Interaction that Affects Tumor Development
- Tumor Suppressor Function of Mig-6 and its Limitation of EGFR Signaling is Lost in Some Cases of Glioblastoma Multiforme
Resources For Glioblastoma
Astrocytomas are classified according to a grading system developed by the World Health Organization (WHO). Astrocytomas come in four grades based upon how fast the cells are reproducing and that likelihood that they will infiltrate nearby tissue. Grades I or II astrocytomas are nonmalignant and may be referred to as low-grade. Grades III and IV astrocytomas are malignant and may be referred to as high-grade astrocytomas. Grade III astrocytomas are known as anaplastic astrocytomas. Grade IV astrocytomas are known as glioblastoma multiforme.
